Research Projects
Implantation of the human embryo into the uterine wall represents a critical developmental milestone; up to 50% of pregnancies/fetal development fail during this important peri-implantation period. The exact causes of early implantation failure remain largely unknown given the technical and ethical limitations of studying this developmental time period in the human. Our goal is to understand molecular and cellular mechanisms of peri-implantation human development, a period referred to as the “black box” of human embryogenesis, using animal as well as hPSC-based in vitro organoid models that enable us to mechanistically probe human peri-implantation embryogenic events, in particular the formation of the epiblast structure and the pro-amniotic cavity (amniotic fluid reservoir), as well as amniogenesis (e.g., hPSC-cyst and hPSC-amnion shown in the figure).
Major goals of the Taniguchi lab are to advance the understanding of how:
The apicosome drives the formation of the epiblast/amniotic lumen
Amnion fate is determined
Clinically, we expect that these explorations will result in new diagnostic and therapeutic strategies for infertility.
Apicosome and epiblast formation
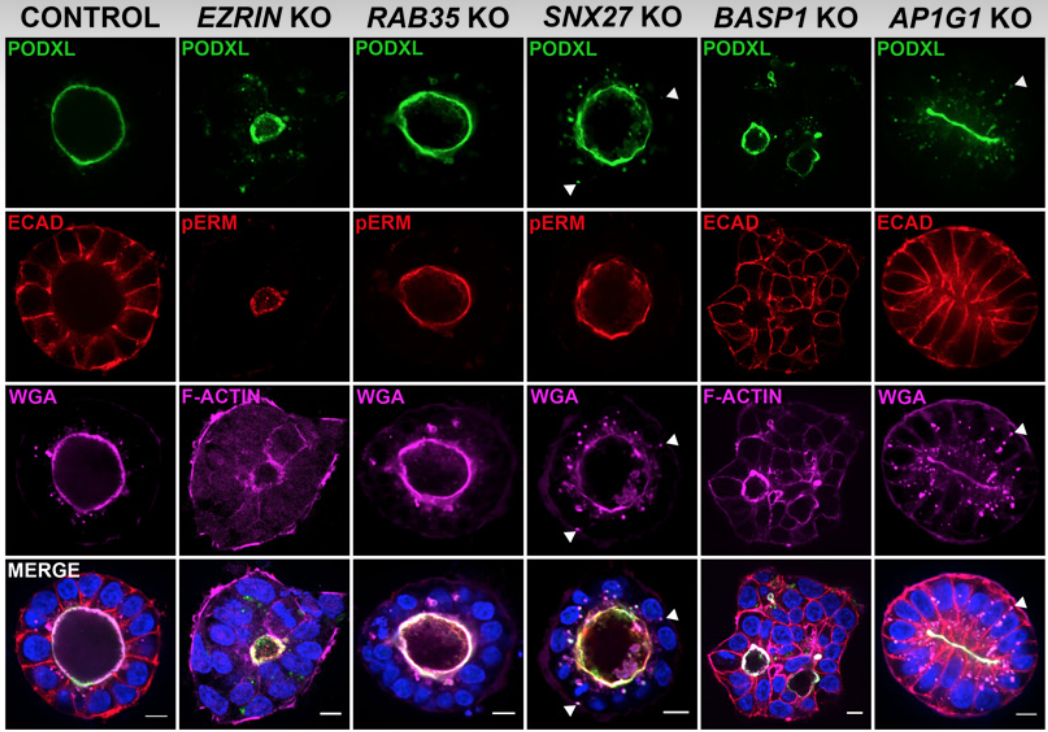
Upon implantation, the aggregate of epiblast cells undergoes a dramatic reorganization: cells polarize along their apico-basal axis and adopt a rosette-like structure with a central shared lumen (epiblast/pro-amniotic cavity). Recently, epiblast cell polarization and epiblast/pro-amniotic cavity formation have been investigated using hPSC-cyst (Taniguchi et al., Stem Cell Reports 2015), and, we found that the steps of hPSC-cyst formation are similar to epiblast lumen formation in vivo, in which seemingly unpolarized collections of hPSC initiate radial organization then a central lumen formation. Mechanistically, this process is driven by an apicosome – an apically charged organelle with characteristics of an intracellular lumen, complete with microvilli, primary cilium and increased concentration of calcium (Taniguchi, Shao et al., Journal of Cell Biology 2017). Our data show that the apicosome functions as a pre-made lumenal precursor that fast-tracks lumen formation during pro-amniotic cavity development, and we recently demonstrated a novel requirement of the AP-1 clathrin adaptor complex in apicosome formation (Wang et al., Science Advances 2021). We currently have two major objectives in our apicosome research:
Identify building blocks of the apicosome and polarized epithelium at global proteomic levels
Elucidate molecular machineries and signaling events controlling apicosome formation and trafficking.
Students and postdocs will have a unique opportunity to engage in efforts to screen for proteins that regulate apicosome formation and lumenogenesis, and to undertake new and exciting projects examining fundamental aspects of apicosome biology. See the review paper by Amber (Carleton et al. Seminars in Cell and Developmental Biology 2022) to catch up with recent advances in mammalian epiblast morphogenesis.
Amnion is a vital component of fetal development. Amnion is an amniotic fluid reservoir, and functions to provide physical protection to the fetus, as well as to aid in development (nutrients, hormones, etc.). During human development, amnion forms around 7-8 dpc (days post coitum) immediately following the formation of the pro-amniotic/epiblast cavity. To study these early events, we developed culture conditions that allow directed differentiation of hPSC-cyst toward amnion lineage. In these conditions, hPSC-cysts undergo progressive cellular flattening, loose pluripotency, and acquire morphological (squamous) and transcriptomic features consistent with amniotic (hPSC-amnion, Shao, Taniguchi et al., Nature Materials 2017). The hPSC-amnion model enables mechanistic analyses of amnion development, and we have already discovered that the activation of the bone morphogenetic protein (BMP) signaling pathway (downstream of mechanical cue in the 3D culture condition) is critical for the initial step of amnion specification by activating an amniotic transcriptional cascade. We recently developed a new and more robust hPSC-based amniogenic system called Glass-3D+BMP, and investigated BMP-dependent transcriptional machineries in depth (Sekulovski et al. eLife 2023). Importantly, our very recent study identified an amnion progenitor-like cell population (Sekulovski et al. bioRxiv 2023). Using state-of-the-art tools such as genome editing and high-throughput RNA sequencing, we are actively investigating:
Amniogenesis
How the mechanical cue results in the activation of BMP signaling
What are the BMP-responsive transcription factors that enable the differentiation toward amniotic lineage from pluripotent cell types (pioneer factor)
Clinically, there are several notable amnion-related conditions that endanger both fetus and mother, such as pre-term premature rupture of fetal membranes (pPROM, premature amnion rupture due to premature weakening of the fetal membrane) and constriction band syndrome (CBS, amputation of fetal extremities as a torn amnion constricts part of the fetus). Exploration of mechanisms regulating amniogenesis will expand our understanding of these pathological conditions.
This is an exciting opportunity for graduate students and postdocs to participate in the discovery of novel amniotic transcription factors, uncover additional mechanisms of amniogenesis, and embark on generating further refined platforms that enable studies of amniogenesis at various developmental stages.
Our Tools